Atoms Of The Same Element
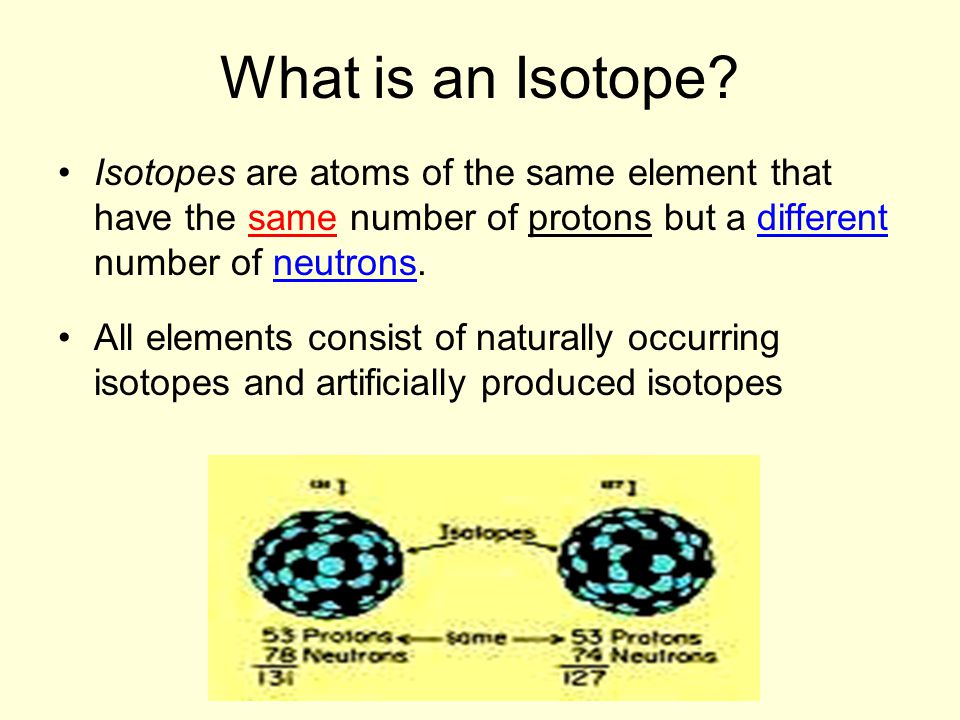
All atoms if the same element have the same number if protons, but the number of neutrons can vary. Atoms with the same number of protons but a different number of neutrons are called isotopes. One example: there are two stable isotopes of chlorine, Cl-35 and Cl-37. Both have 17 protons, but one has 18 and the other 20 neutrons (and s. Isotopes: are atoms of the same element having the same number of protons or the same atomic number but having different numbers of neutrons or different mass numbers. The symbol for an isotope of an atom is written in the form X A Z. The atoms of different elements combine with each other through ionic or covalent bonding to produce compounds this stable form is called The loss of strength in compression which occurs, when there is a gain of strength in tension due to over loading is called? An atom is the smallest amount of a particular element that can be identified as that element. Atoms are made up of three subatomic particles. Protons and neutrons form the nucleus of an atom, and a cloud of electrons orbits the nucleus. Atoms of the same element Atoms of different elements combining to form a molecule.
Article- Atomic model
- Basic properties
- The electron
- The nucleus
- Development of atomic theory
- The beginnings of modern atomic theory
- Studies of the properties of atoms
- Models of atomic structure
- Advances in nuclear and subatomic physics
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program
 and our community of experts to gain a global audience for your work!
and our community of experts to gain a global audience for your work! Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
Most of the atom is empty space. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. Electrons are attracted to any positive charge by their electric force; in an atom, electric forces bind the electrons to the nucleus.
Because of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties. In some respects, the electrons in an atom behave like particles orbiting the nucleus. In others, the electrons behave like waves frozen in position around the nucleus. Such wave patterns, called orbitals, describe the distribution of individual electrons. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells. How to make a paper document into a pdf for macfasrtrek.
Atoms Of The Same Element That Have A Different Number Of Neutrons
This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. For additional information pertaining to nuclear structure and elementary particles, seesubatomic particles.
Atomic model
Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.
As noted in the introduction to this article, an atom consists largely of empty space. The nucleus is the positively charged centre of an atom and contains most of its mass. It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. Other subatomic particles may be found in association with these three types of particles. They can be created only with the addition of enormous amounts of energy, however, and are very short-lived.
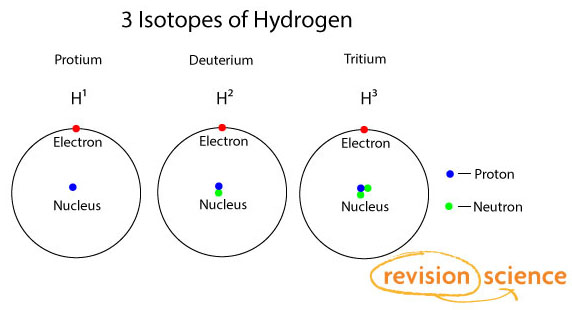
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10−10 metre. The radius of an atom measures 1–2 Å. Compared with the overall size of the atom, the nucleus is even more minute. It is in the same proportion to the atom as a marble is to a football field. In volume the nucleus takes up only 10−14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring nuclear sizes is the femtometre (fm), which equals 10−15 metre. The diameter of a nucleus depends on the number of particles it contains and ranges from about 4 fm for a light nucleus such as carbon to 15 fm for a heavy nucleus such as lead. In spite of the small size of the nucleus, virtually all the mass of the atom is concentrated there. The protons are massive, positively charged particles, whereas the neutrons have no charge and are slightly more massive than the protons. The fact that nuclei can have anywhere from 1 to nearly 300 protons and neutrons accounts for their wide variation in mass. The lightest nucleus, that of hydrogen, is 1,836 times more massive than an electron, while heavy nuclei are nearly 500,000 times more massive.
Basic properties
Atomic number
The single most important characteristic of an atom is its atomic number (usually denoted by the letter Z Illustrator software for mac downloadbrownsearch. ), which is defined as the number of units of positive charge (protons) in the nucleus. For example, if an atom has a Z of 6, it is carbon, while a Z of 92 corresponds to uranium. A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance. Since it is the electrons that determine how one atom interacts with another, in the end it is the number of protons in the nucleus that determines the chemical properties of an atom.
- key people
- related topics
Atomic Structure
Chlorine atom'>Atoms are the basic building blocks of everything around us. They come in different kinds, called elements, but each atom shares certain characteristics in common. All atoms have a dense central core called the atomic nucleus. Forming the nucleus are two kinds of particles: protons, which have a positive electrical charge, and neutrons, which have no charge. All atoms have at least one proton in their core, and the number of protons determines which kind of element an atom is. For example, an oxygen atom has 8 protons. If you were somehow able to change the proton number of this atom to 7, even if everything else remained the same, it would no longer be an oxygen atom, it would be nitrogen. For this reason, we list the different elements by their proton, or atomic, number. The periodic table of elements is a chart of all of the elements that have been discovered so far, in order by their atomic number.
In addition to protons and neutrons, all atoms have electrons, negatively charged particles that move around in the space surrounding the positively-charged nuclear core. Electrons are usually depicted in drawings as much smaller than protons or neutrons because their mass is so much smaller. In fact, electron mass is so small that it is not counted in an atom’s mass. However, the charge strength of a single electron is equal to that of a single proton, and despite their small mass, electrons are important for balancing the charge of an atom. Unless specifically stated otherwise, atoms always have the same number of electrons as protons; therefore, you can find the electron number by looking at the atomic number. But unlike protons, the number of electrons can and does change without affecting the kind of element an atom is!
We now know how to find the number of protons and the number of electrons for a given atom, but what about neutrons? How many neutrons do atoms of a given element have? It is NOT always the same as the number of protons and electrons. For example, hydrogen has one proton and one electron, but it doesn’t have any neutrons at all! We determine this by looking at the atomic mass. Even though an atom is so small it would take almost a million for you to see even a tiny dot on your computer screen, each tiny atom definitely has mass and occupies space. This mass comes from the nucleus. Each proton and neutron has about the same amount of mass, measured in daltons, or atomic mass units (amus). Because the unit of measure is defined by one proton, 1 proton = 1 neutron = 1 dalton = 1 amu. Electrons do have some mass, but it is almost 2000 times less than the mass of a proton. There aren’t enough electrons in any of the atoms we know about to affect the total mass; therefore, the total mass is equal to the sum of the protons and the neutrons in an atom.
Because we can find the number of protons and the atomic mass of an atom by looking at its element information in the periodic table, we can calculate the number of neutrons in that atom by subtracting the number of protons from the atomic mass.
When the number of neutrons is different for individual atoms of the same element, each atom is called an isotope. When you read a periodic table, the atomic mass listed is the average atomic mass for all of the isotopes of that element found in nature. For example, carbon has an atomic mass of 12.01 in the periodic table. Carbon can’t have 6.01 neutrons because you can’t have part of a neutron. The value exceeds 6 because, while most carbon atoms have 6 neutrons, some carbon atoms are found with 7 neutrons and others with 8 neutrons. For our purposes, we round the atomic mass to the nearest whole number to calculate the number of neutrons.
Atomic Structure
This video illustrates how atoms and their components work together.
Valence Electrons
Atoms Of The Same Element Can Have Different Properties
Now that you’ve had a chance to work with atoms in general, let’s dig a little deeper. Electrons remain in an atom because of their attraction to the protons’ positive charge, but they are not as tightly associated with the atom as either protons or neutrons. Electrons are complicated particles because they have a lot of space to occupy in an atom, and yet they are also tied to a specific area within that atom. Although the drawings we have been working with show the nucleus as a medium-sized, visible object in the center of an atom, it is actually very tiny, and most of an atom is the space around the nucleus in which the electrons move.
Because of their shared negative charge, electrons repel one another if they get too close. At the same time, electrons are attracted to the positive charge of the nucleus. The details of the energy and position of electrons can get really complicated, but we will focus only on what we need to understand to study the molecules of life.
Sulfur atom'>Electrons are arranged in energy shells (also known as electron shells) around the atomic nucleus. Although electrons have plenty of space, they all want to be closest to the positive nuclear charge that is attracting them. At the same time, the electrons repel one another because of their negative charge, and only a few can get close to the nucleus at any given time. Practically speaking, only two electrons can fit in the three-dimensional space closest to the nucleus. This space is called the first energy shell. If there are three electrons in an atom, the first two will be found in the first energy shell. The third electron will have to settle for the second energy shell, a three-dimensional space a little farther from the nucleus, where it will be alone. In this example, the lone electron is called a valence electron, and the outermost energy shell that contains any electrons is called the valence shell.
Atoms Of The Same Element Can Differ
Aluminum atom'>The second energy shell is big enough to hold as many as eight electrons, grouped in pairs inside four electron orbitals, or spaces where electrons spend most of their time. This means if there is only one electron in the second energy shell, there is a lot of extra space left.

When an energy shell is incompletely filled, the electron(s) in that shell are not as stable and are more likely to react. For this reason, atoms tend to react with other atoms in ways that will fill or empty their valence shell to gain the stability of a full outermost energy shell. Atoms can do this by gaining or losing electrons to become ions or by sharing electrons with other atoms to form stable associations.
Using electron number and energy shells, we can determine the number of valence electrons for any given atom and its expected level of reactivity. As you work with the example below, you should remember that although we draw energy shells as circles around an atomic nucleus, this is not meant to represent an actual electron path. The concentric circle style of drawing energy shells is meant to represent the average distance electrons in that energy shell are orbiting the nucleus. In reality, electrons do not move in a circular orbit as depicted in the drawing, but travel much more complicated pathways around an atomic nucleus.
Difference Between Atom And Element
Build an Atom
Atoms Of The Same Element
Use this activity to practice reading the periodic table to create several atoms.
What is the Valence?
Atoms Of The Same Element Examples
In this activity, you will calculate the number of valence electrons in atoms using the periodic table of elements.
