Ferro Magnetism
Definition of ferromagnetic: of or relating to substances with an abnormally high magnetic permeability, a definite saturation point, and appreciable residual magnetism and hysteresis Other Words from ferromagnetic Example Sentences Learn More about ferromagnetic Other Words from ferromagnetic. Of or characteristic of substances such as iron, nickel, or cobalt and various alloys that exhibit extremely high magnetic permeability, a characteristic saturation point, and magnetic hysteresis. Ferromagnetism will provide a useful resource to any electrical engineer, physicist, researcher or designer, interested in the field of magnetics. Also of Interest Electromagnetics History, Theory, and Applications by Robert S. Elliott, UCLA A Classic Reissue in the IEEE Press Series on Electromagnetic Waves A handy reference for engineers.
Ferromagnetic materials are also characterized by being made up of clusters of 10 17 to 10 21 atoms called magnetic domains, that all have their magnetic moments pointing in the same direction. The moments of the domains is random in unmagnetized materials, and point in the same direction in magnetized materials. Ferromagnetism meaning: 1. The type of magnetism (= the force that makes certain objects move towards it) that iron has 2.
Also found in: Dictionary, Thesaurus, Medical, Wikipedia.ferromagnetism:
see magnetismmagnetism,force of attraction or repulsion between various substances, especially those made of iron and certain other metals; ultimately it is due to the motion of electric charges.
.....Click the link for more information..
Ferromagnetism
A property exhibited by certain metals, alloys, and compounds of the transition (iron group), rare-earth, and actinide elements in which, below a certain temperature called the Curie temperature, the atomic magnetic moments tend to line up in a common direction. Ferromagnetism is characterized by the strong attraction of one magnetized body for another.
Atomic magnetic moments arise when the electrons of an atom possess a net magnetic moment as a result of their angular momentum. The combined effect of the atomic magnetic moments can give rise to a relatively large magnetization, or magnetic moment per unit volume, for a given applied field. Above the Curie temperature, a ferromagnetic substance behaves as if it were paramagnetic: Its susceptibility approaches the Curie-Weiss law. The Curie temperature marks a transition between order and disorder of the alignment of the atomic magnetic moments. Some materials having atoms with unequal moments exhibit a special form of ferromagnetism below the Curie temperature called ferrimagnetism. SeeCurie temperature, Curie-Weiss law, Electron spin, Ferrimagnetism, Magnetic susceptibility, Paramagnetism
The characteristic property of a ferromagnet is that, below the Curie temperature, it can possess a spontaneous magnetization in the absence of an applied magnetic field. Upon application of a weak magnetic field, the magnetization increases rapidly to a high value called the saturation magnetization, which is in general a function of temperature. For typical ferromagnetic materials, their saturation magnetizations, and Curie temperatures, SeeMagnetization.
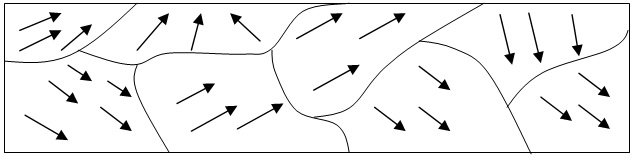
Small regions of spontaneous magnetization, formed at temperatures below the Curie point, are known as domains. As shown in the illustration, domains originate in order to lower the magnetic energy. In illus. b it is shown that two domains will reduce the extent of the external magnetic field, since the magnetic lines of force are shortened. On further subdivision, as in, this field is still further reduced.

Another way to describe the energy reduction is to note that the interior demagnetizing fields, coming from surface poles, are much smaller in the long, thin domains of illus. c than in the “fat” domain of illus. a.

The question arises as to how long this subdivision process continues. With each subdivision there is a decrease in field energy, but there is also an increase in Heisenberg exchange energy, since more and more magnetic moments are aligning antiparallel. Finally a state is reached in which further subdivision would cause a greater increase in exchange energy than decrease in field energy, and the ferromagnet will assume this state of minimum total energy.
Materials easily magnetized and demagnetized are called soft; these are used in alternating-current machinery. The problem of making cheap soft materials is complicated by the fact that readily fabricated metals usually have many crystalline boundaries and crystal grains oriented in many directions. The ideal cheap soft material would be an iron alloy fabricated by some inexpensive technique which results in all crystal grains being oriented in the same or nearly the same direction. Various complicated rolling and annealing methods have been discovered in the continued search for better grain-oriented or “cube-textured” steels.
Materials which neither magnetize nor demagnetize easily are called hard; these are used in permanent magnets. A number of permanent-magnet materials have enjoyed technological importance. The magnet steels contain carbon, chromium, tungsten, or cobalt additives, serving to impede domain wall motion and thus to generate coercivity. Alnicos are aluminum-nickel-iron alloys containing finely dispersed, oriented, elongated particles precipitated by thermal treatment in a field. Hard ferrite magnets are based on the oxides BaFe12O19 and SrFe12O19. Hard ferrite magnets are relatively inexpensive and are used in a great variety of commercial applications. Rare earth–transition metal materials whose rare-earth component provides huge magnetocrystalline anisotropy can be translated into large coercivity in a practical magnet, while the magnetization arises chiefly from the transition-metal component. Examples include samarium-cobalt magnets based on the SmCo5 or Sm2Co17 intermetallic compounds.
Ferromagnetism
a magnetic state of, as a rule, crystalline substances that is characterized by parallel orientation of the atomic magnetic moments. Parallel orientation of the magnetic moments (Figure 1) is established at temperatures T below a critical temperature θ (seeCURIE POINT) and is due to the positive energy of the electron-electron exchange interaction (seeMAGNETISM). Ferromagnetic ordering of the magnetic moments in crystals—that is, collinear or noncollinear atomic magnetic structure—is directly observed and investigated by the methods of magnetic neutron diffraction analysis. Substances in which the atomic magnetic moments are ferromagnetically ordered are called ferromagnets. The magnetic susceptibility X of ferromagnets is positive (χ > 0) and may be as high as 104–105 gauss/oersted (G/Oe); the magnetization J or induction B = H + 4πJ of ferromagnets increases nonlinearly with increasing magnetic field strength H (Figure 2) and, in fields of 1–100 Oe, reaches a limiting value Js, which corresponds to magnetic saturation. The value of J also depends on the previous magnetic history of a specimen. This makes the dependence of J on H ambiguous; that is, magnetic hysteresis is observed.
The manifestations of ferromagnetism in single crystals and polycrystals may differ substantially. Magnetic anisotropy (Figure 3), which is the difference in magnetic properties in different crystallographic directions, is observed in ferromagnetic single crystals. In polycrystals with a random distribution of the crystal grain orientations, magnetic anisotropy is, on the average, absent in a specimen; however, when the orientations are nonuniformly distributed, anisotropy may be observed as texture.
The magnetic and other physical properties of ferromagnets have a specific dependence on temperature T. The saturation magnetization Js has a maximum value at T = 0°K and decreases monotonically to zero at T = θ (Figure 4). Above θ, a ferromagnet becomes a paramagnet (seePARAMAGNETISM) or, in certain cases (the rare-earth metals), an antiferromagnet. At H = 0, the transition to a paramagnet or an antiferromagnet is, as a rule, a second-order phase transition. The temperature dependence of the magnetic permeability μ, or susceptibility χ of ferromagnets has a pronounced maximum near θ. At T > θ, the susceptibility χ usually obeys the Curie-Weiss law. When ferromagnets are magnetized, their size and shape change (seeMAGNETOSTRICTION). The magnetization curves and hysteresis loops therefore depend on the external stresses. Anomalies are also observed in the value and temperature dependence of the elastic constants and the coefficients of linear and cubical expansion. Upon adiabatic magnetization and demagnetization, ferromagnets undergo a change in temperature (seeMAGNETIC COOLING). The specific features of the nonmagnetic properties of ferromagnets are exhibited most clearly near T = θ.
Since the spontaneous magnetization of ferromagnets is preserved up to T = θ and since the temperature θ may be as high as ~103°K in typical ferromagnets, kθ ≈ 10–13 erg, where k is the Boltzmann constant. This means that the interaction energy responsible for the ferromagnetic ordering of the atomic magnetic moments in a crystal should also be of the order of 10–13 erg per pair of adjacent magnetic atoms. Such an energy value can result only from electrical interaction between electrons, as the magnetic interaction energy of the electrons of two adjacent atoms in a ferromagnet does not, as a rule, exceed 10–16 erg and can therefore ensure a Curie temperature of only ~1°K (ferromagnets with the magnetic dipole interaction also exist). In the general case, magnetic interactions in ferromagnets determine the magnetic anisotropy of the substances. Classical physics could not explain how the electrical interaction can result in ferromagnetism. It was only the use of quantum mechanics that made it possible to understand the close intrinsic relationship between the resulting magnetic moment of a system of electrons and the electrostatic interaction of the electrons, which is usually called the exchange interaction.
The presence of permanent magnetic moments—that is, magnetic moments not dependent on H—of the electron shells of the atoms in ierromagnets is a necessary condition for ferromagnetism; the magnetic moments may be spin moments, orbital moments, or both. This condition is fulfilled in crystals consisting of atoms of transition elements, that is, atoms with incomplete inner electron shells. The following four main cases are distinguished: (1) metal crystals (pure metals, alloys, and intermetallic compounds) based on transition elements with incomplete d shells, chiefly the 3d shell in iron-group elements; (2) metal crystals based on transition elements with incomplete f shells, that is, rare-earth elements with an incomplete 4f shell; (3) nonmetallic crystalline compounds if at least one component consisting of transition elements with an incomplete d or f shell is present; (4) highly dilute solutions of atoms of transition metals with an incomplete d or f shell in a diamagnetic metallic matrix. The atomic magnetic order in these four cases is due to the exchange interaction.
In nonmetallic substances [case (3)], the interaction is most often a superexchange interaction in which the electrons in incomplete d or f shells in the nearest adjacent paramagnetic ions are magnetically ordered with the active participation of electrons in the closed outermost shells of nonmagnetic ions, such as O2–, S2–, or Se2–. The nonmagnetic ions are usually located between the magnetic ions (see). In this case, as a rule, antiferromagnetic order occurs. The occurrence of such order results either in antiferromagnetism, if the total magnetic moment of all the ions is equal to zero in each unit cell of the crystal, or in ferrimagnetism, if the total magnetic moment is not equal to zero. Cases are possible in which the interaction in nonmetallic crystals is ferromagnetic in nature; that is, all the atomic magnetic moments are parallel. Examples of such crystals include EuO, Eu2SiO4, and CrBr3.
The presence of a system of conduction electrons is common to crystals of the types described in cases (1), (2), and (4). Although magnetizing exchange interactions exist in such systems, there is, as a rule, no magnetic order, and Pauli paramagnetism occurs if it is not suppressed by the stronger diamagnetism of the ionic lattice. If magnetic order does occur, its origin is different in cases (1), (2), and (4). In case (2), the magnetic 4 f shells have a very small radius in comparison with the lattice constant. Therefore, in this case, exchange coupling is impossible, even between nearest-neighbor ions. Such a situation is also characteristic of case (4). In both case (2) and case (4), exchange coupling is indirect and accomplished by conduction electrons. In ferromagnets corresponding to case (4)—in contrast to cases (1), (2), and (3)—the magnetic order is not necessarily associated with the crystalline atomic order. Such ferromagnets are often magnetically amorphous systems with ions that are randomly distributed throughout the crystal lattice and that have atomic magnetic moments; such systems are called spin glasses.
Finally, in crystals corresponding to case (1), the electrons involved in the creation of atomic magnetic order are the former 3d and 4f electrons of isolated atoms. In contrast to the 4f shells of rare-earth ions, shells that have a very small radius, the 3d electrons of Fe-group atoms are closer to the periphery of the atom and form a conduction band. Together with the 4s electrons, the
3d electrons form a general system of conduction electrons. However, in contrast to nontransition metals, the system of conduction electrons in metals with an incomplete d shell has a much higher density of energy levels. This higher density contributes to the action of the exchange forces and leads to the occurrence of the magnetized state in Fe, Co, Ni, and the numerous alloys of these metals.
Specific theoretical calculations of the various properties of ferromagnets are performed both in the quasi-classical phenomenological approximation and by means of more rigorous quantum-mechanical atomic models. In the quasi-classical case, the exchange interaction that results in ferromagnetism is taken into account by introducing an effective molecular field (B. L. Rozing, 1897; P. Weiss, 1907). The energy U of the molecular field is proportional to the square of J:
U = – NA (Js/Js0)2
where N is the number of magnetic atoms in the specimen, A is the molecular field constant (A > 0), and Js0 is the saturation magnetization at a temperature of absolute zero. A quantum-mechanical refinement of this treatment of ferromagnetism was made after the discovery of the electrical exchange nature of the constant A (Ia. I. Frenkel’ and W. Heisenberg, 1928). In particular, at low temperatures (T << θ) a more exact quantum calculation was performed by F. Bloch in 1930. Bloch’s calculation showed that the decrease in the spontaneous magnetization Js0 of a ferromagnet with increasing temperature may be described in the first approximation as the occurrence of elementary magnetic excitations, or quasiparticles called spin waves or magnons. Each magnon reduces Js0 by the value of the magnetic moment of one lattice point. The number of magnons increases in proportion to T3/2 as the ferromagnet is heated. Therefore, the temperature dependence of Js has the form
Js = Js0 (1 – αT3/2)
where the coefficient α is of the order of 10–6°K–3/2 and depends on an exchange-interaction parameter.
In the absence of an applied magnetic field (H = 0), the demagnetized state corresponds to the thermodynamically stable state of a macroscopic ferromagnetic specimen; otherwise, magnetic poles that generate a demagnetizing field H0 with which a large positive energy is associated would, as a rule, occur on the surface of the specimen. At the same time, the exchange interaction tends to produce magnetic order with J ≠ 0. As a result of the conflict between these opposite tendencies, a ferromagnetic specimen is divided into domains, that is, into regions of uniform magnetization. The theory of ferromagnetism qualitatively defines the domain size and shape, which depend on the competition between different interactions in a ferromagnetic crystal (L. D. Landau and E. M. Lifshits, 1935). The equilibrium structure of the domains at J = 0 corresponds to closed magnetic fluxes within the specimen. Transition zones of finite thickness, in which Js continuously changes direction, exist between domains. Positive energy is expended on the formation of these zones, but it is less than the field energy of H0 that would be produced in the absence of the domains. At some critically small size of ferromagnetic specimens, the formation of several domains in the specimens may become energetically unfavorable. Such small ferromagnetic particles will then be uniformly magnetized at T < θ; these particles are called single-domain particles.
Magnetization curves and hysteresis loops for ferromagnets are determined by changes in the volume of domains with different orientations of Js. Such changes are due to displacement of the domain boundaries and to rotation of the vectors Js, of the domains (seeMAGNETIZATION). The magnetic susceptibility of ferromagnets may be approximately represented as the sum χ = χdis + χrot. Analysis of the magnetization curves J(H) shows that χdis ≫ χrot in weak fields and χrot ≫ χdis in strong fields (after a steep rise of the curve). The magnetization processes and the distribution of magnetization in magnetic thin films are of a special nature. Because of the sensitivity of domain structure and magnetization processes to crystal structure, the general quantitative theory of the magnetization curves of ferromagnets remains incomplete. Qualitative physical concepts are ordinarily used to determine the relation J(H), and a rigorous quantitative calculation is possible only in the case of ideal single crystals in the region where χrot ≫ χdis (N. S. Akulov, 1928).
The theory of magnetization curves and hysteresis loops is important for the development of new magnetic materials and the improvement of existing materials.
The relation between ferromagnetism and many nonmagnetic properties of a substance makes it possible to obtain information on various detailed specific features of the electronic structure of crystals from measurements of magnetic properties. Therefore, ferromagnetism is studied at the electronic and nuclear levels by using, for example, electron ferromagnetic resonance, nuclear magnetic resonance, the Mössbauer effect, and the scattering of corpuscular radiation by various types of ferromagnetic crystals with allowance for the influence of the magnetic moments of the interacting particles.
In the 1970’s, interesting relationships were found between ferromagnetism and elementary-particle physics and astrophysics. In this regard, we should mention the study of positron annihilation, the formation of muonium and positronium (seePOSITRON), and the scattering of muons in ferromagnetic substances, as well as the problem of the magnetism of neutron stars (pulsars) in astrophysics.
REFERENCES
Akulov, N. S. Ferromagnetizm. Moscow-Leningrad, 1939.Bozorth, R. Ferromagnetizm. Moscow, 1956. (Translated from English.)
Vonsovskii, S. V., and Ia. S. Shur. Ferromagnetizm. Moscow-Leningrad, 1948.
Dorfman, Ia. G. Magnitnye svoistva i stroenie veshchestva. Moscow, 1955.
Turov, E. A. Fizicheskie svoistva magnitouporiadochennykh kristallov. Moscow, 1963.
Teoriia ferromagnetizma metallov i splavov: Sb. Moscow, 1963. (Translated from English.)
Akhiezer, A. I., V. G. Bar’iakhtar, and S. V. Peletminskii. Spinovye volny. Moscow, 1967.
Turov, E. A., and M. P. Petrov. Iadernyi magnitnyi rezonans v ferro-i antiferromagnetikakh. Moscow, 1969.
Sverkhtonkie vzaimodeistviia v tverdykh telakh. Moscow, 1970. (Translated from English.)
Vonsovskii, S. V. Magnetizm. Moscow, 1971.
Becker, R., and W. Döring. Ferromagnetismus. Berlin, 1939.
Kneller, E. Ferromagnetismus. Berlin, 1962.
Magnetism, vote. 1–4. New York–London, 1963–66.
Amorphous Magnetism. London–New York, 1973.
Goodenough, J. B. Magnetism and the Chemical Bond. New York–London, 1963.
ferromagnetism
[¦fe·rō′mag·nə‚tiz·əm] (solid-state physics)Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Link to this page:

Ferromagnetic materials or substances are invented by a French physicist Louis Eugene Felix Neel. He was born on 22nd November 1904 in Lyon & died on 17th November 2000 Brive-la-Gaillarde. He studied at Strasbourg University & got a Nobel prize in physics. There are several ferromagnetic material manufacturing companies available like Dexter Magnetic Technologies founded in 1951 in Elk Grove Village, Digi Key Electronics founded in 1972 in Thief River Falls, RS components founded in 1937 in Corby by Waring and P.M.Sebestyen, Star Trace Private Limited established in 1985 in Tamilnadu, Shields Company Magnetics in Culver city, Magnum Magnetics Corporation in Marietta, Alliance LLC, Arnold Magnetic Technologies, International Magna Products, Master Magnetics are some of the top magnetic manufacturers.
What are the Ferromagnetic Materials?
In some materials, the permanent atomic magnetic moments have a strong tendency to align themselves even without any external field. These materials are said to be ferromagnetic materials. Some of the examples of ferromagnetic materials are cobalt, iron, nickel, gadolinium, dysprosium, permalloy, awaruite, wairakite, magnetite, etc. There are many ferromagnetic materials, some of the ferromagnetic materials lists is shown in the below table.
| S.NO | Ferromagnetic Materials | Curie Temperature | Melting Point | Boiling Point | Atomic Number | Density |
| 1. | Cobalt | 1388 | 1768K | 3200K | 27 | 8.90g/cm3 |
| 2. | Iron | 1043 | 1811K | 3134K | 26 | 7.874g/cm3 |
| 3. | Nickel | 627 | 1728K | 3003K | 28 | 8.908g/cm3 |
| 4. | Neodymium Magnet | 593 | 1297 K | 3347 K | 60 | 0.275 lbs. per cubic inch |
| 5. | Chromium dioxide | 386 | >3750C | 40000C | 24 | 4.89g/cm3 |
| 6. | Gadolinium | 292 | 1585K | 3273K | 64 | 7.90g/cm3 |
| 7. | Terbium | 219 | 1629K | 3396K | 65 | 8.23g/cm3 |
| 8. | Dysprosium | 88 | 1680K | 2840K | 66 | 8.540g/cm3 |
1). Cobalt: The Cobalt is invented by Georg Brandt in 1739. He was born on 26th June 1964 in Riddarhyttan and died in Stockholm on 29th April 1768. It is one type of ferromagnetic material found in the earth’s crust. It is represented by a symbol CO in the periodic table and its atomic number is 27.
2). Iron: An iron is the one type of chemical element which is found in the earth’s crust and it is generally represented by a symbol Fe. The color of iron is silvery grey and the atomic number is 26 in the periodic table. The first electric iron is invented in 1882 by Henry W Seeley, which is used to iron the clothes. The Henry W Seeley born on 20th May 1861 in Newyork and died on 20th May 1943.
3). Nickel: The chemical element nickel is also found in the earth’s crust and it is represented by a symbol Ni. The atomic number of nickels is 28 in the periodic table and the color of nickel is silvery white. This metal is invented by Axel Fredrik Crostedt, he was born in Sweden on 23rd December 1722 and died on 20th May 1943.
4). Neodymium Magnet: It is one type of strong and permanent magnet but it is found in the earth crust rarely and the color of neodymium is silvery white. It is also called as NIB or Neo or NdFeB magnet and the formula of neodymium magnet is Nd2Fe14B. This metal is invented by Carl Auer Von Welsbach, he was born in Austria on 1st September 1858 and died on 4th August 1929.
5). Chromium dioxide: The chemical formula of chromium dioxide is CrO2, it is insoluble in water and it is also called as Chromium (iv) oxide. The other names of Chromium dioxide are Carolyn and magtrieve. The metal chromium is discovered by Louis Nicolas Vauquelin, he was born in Austria on 16th May 1763 and died on 14th November 1829 in France.
Ferromagnetism And Antiferromagnetism
6). Gadolinium: The Gadolinium is one type of chemical element, which is represented by a symbol Gd. The atomic number of gadolinium is 64 in the periodic table. The metal gadolinium is invented by Paul-Emile Lecoq de Boisbaudran (18th April 1838 – 28 May 1912) in France and Jean Charles Galissard de Marignac (24th April 1817 – 15th April 1894) in Switzerland.
7). Terbium: The Terbium is also one type of chemical element, which is represented by a symbol Td. It is invented by Carl Gustaf Mosander in 1843 and it is found in earth crust rarely. This chemical element is invented by Carl Gustaf Mosander in 1843. He was born on 10th September 1797in Kalmar and died on 15th October 1858 in Stockholm County.
8). Dysprosium: The Dysprosium is one type of ferromagnetic material, which is identified by Paul Emile Lecoq de Boisbaudran in 1886. He was born on 18th April 1838 and died on 28th May 1912 in France. The atomic number of gadolinium is 66 in the periodic table.
Ferromagnetism Of Iron Oxide
Types of Ferromagnetic Materials
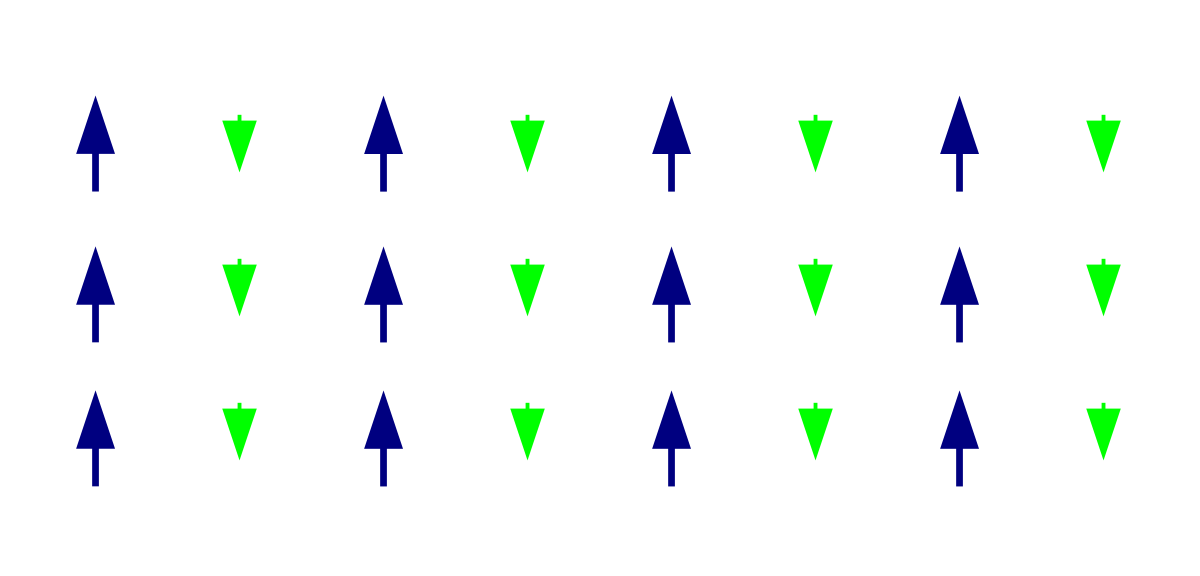
There are two types of ferromagnetic materials they are un-magnetized ferromagnetic material and magnetized ferromagnetic material. The classification of ferromagnetic material is shown in the below figure
Ferromagnetismus
1). Un-magnetized Ferromagnetic Material
In every un-magnetized ferromagnetic material, the atoms form domains inside the material. The different domains have different directions of the magnetic moment. Hence the material remains un-magnetized. The un-magnetized ferromagnetic material shown in the below figure
2). Magnetized Ferromagnetic Material
By applying an external magnetic field to the domains of un-magnetized ferromagnetic, the domains will rotate and aligns in the direction of the magnetic field, because of the domain character of ferromagnetic material even if a small magnetic field is applied gives rise to large magnetization. The magnetic field is much larger than the magnetic field in such material. The magnetic moments of domains are parallel to the magnetic field in ferromagnetism because these domains are also aligning in the same direction.
This is the explanation of the un-magnetized ferromagnetic material and magnetized ferromagnetic material with the diagrams.
Properties of Ferromagnetic Materials
The properties of ferromagnetic material are
- The ferromagnetic substances are strongly attracted by the magnetic field
- These substances show the permanent magnetism even in the absence of magnetic field
- The ferromagnetic substances changes to paramagnetic when the substances are heated at high temperature.
Reason: This is due to the randomization of domains on heating
- All the domains are aligned in a parallel direction
Advantages
The advantages of Ferromagnetic materials are
- Resistance is high
- Cheap
- Hysteresis loss is low
- Electrical resistivity is high,
- Coercivity is low
- High permeability.
- It can operate up to 3000 C temperature
- Stability of ferromagnetic materials are good
Disadvantages
The main disadvantage of Ferromagnetic materials is
- Generates week magnetic field
Applications
The applications of the ferromagnetic materials are
- Electromagnets
- Magnetic tape recording
- Hard drives
- Telephones
- Loudspeakers
- Electric motors
- Hard disk
- Magnetic Storage
This article describes the list of Ferromagnetic Materials and the explanation of each material, applications, advantages, and disadvantages. Here is a question for you which is the best ferromagnetic material and why?
